Learn how pharmacy systems correctly identify generic and brand drugs using NDC codes, TE ratings, and FDA guidelines. Best practices for safe, cost-effective medication substitution.
Read MoreArchive
Sleep Apnea and Its Impact on Heart Rhythm Disorders
Explore how sleep apnea raises the risk of heart rhythm disorders, the science behind it, and practical steps to diagnose and treat both conditions.
Where to Find Detailed Side Effect Information for Your Medications
Learn where to find accurate, up-to-date side effect information for your medications using FDA-approved sources like DailyMed, MedlinePlus, VigiAccess, and OnSIDES. Avoid outdated or biased tools.
State vs Federal Law: How Substitution Rules for Lawyers Differ and Why It Matters
State and federal courts have completely different rules for switching lawyers. Mixing them up can cost you time, money, and your case. Here's how to navigate the legal minefield and avoid costly mistakes.
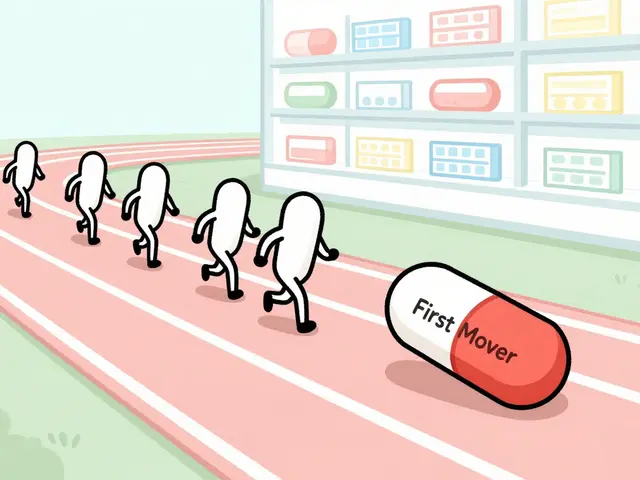
First-Mover Advantage: How First Generic Drug Makers Dominate the Market
The first generic drug maker to launch after a patent expires captures up to 90% of the market - and keeps most of it for years. Here’s how the Hatch-Waxman Act creates lasting dominance.
Medication Reformulations: Why Drug Companies Change Formulas and What It Means for You
Medication reformulation changes how drugs are made to improve how they work - without changing the active ingredient. Learn why companies do it, how it affects you, and what to watch for when your prescription changes.