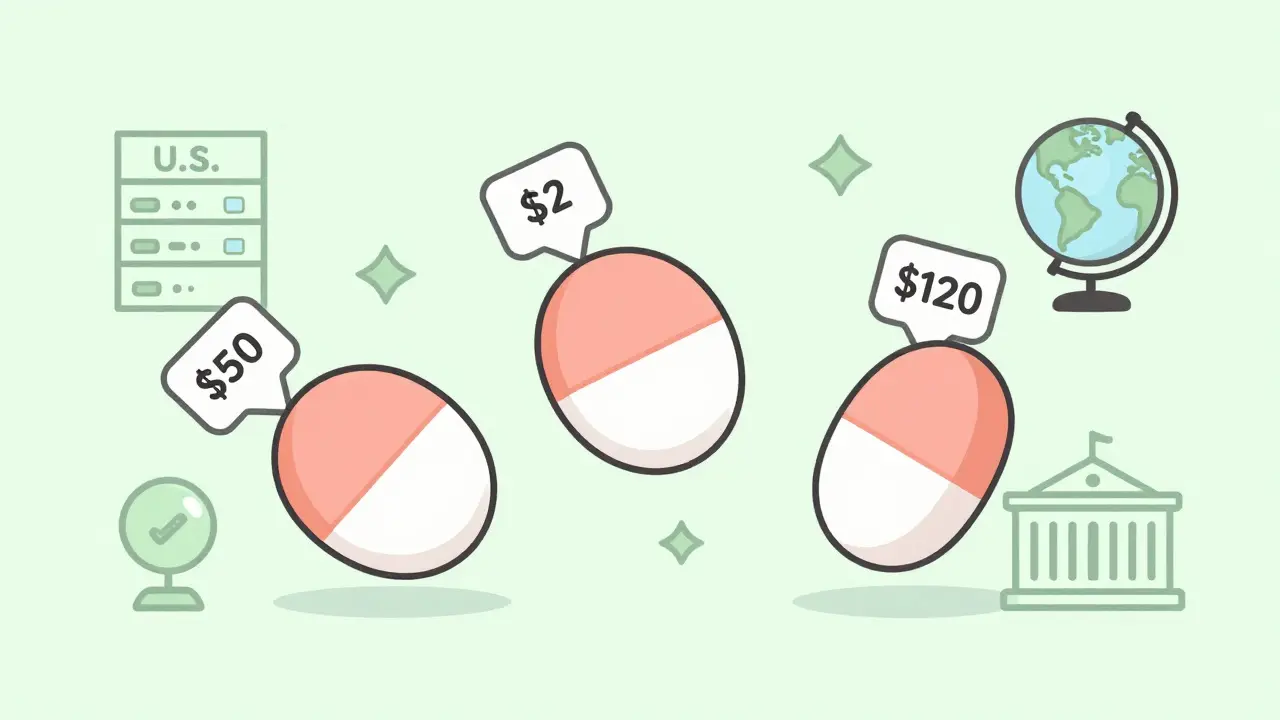
Same pill. Same active ingredient. Same dosage. But in the U.S., it might cost $50. In India, $2. In Switzerland, $120. Why does this happen? It’s not about quality alone. It’s not about cheating. It’s about systems - how countries regulate, pay for, and trust generic drugs.
What Exactly Is a Generic Drug?
A generic drug is a copy of a brand-name medication. Once the original patent expires, other manufacturers can produce the same chemical compound. They don’t need to repeat expensive clinical trials. Instead, they prove their version works the same way in the body - called bioequivalence. That means the drug gets absorbed at the same rate and to the same extent as the brand. It’s not a cheaper version. It’s the same medicine, just without the marketing budget.
But here’s the catch: not every country approves the same generics. Some allow dozens of makers to compete. Others let only one or two in. Some regulators inspect factories without warning. Others give manufacturers a heads-up. That changes everything.
The U.S.: High Volume, Strange Prices
The United States leads the world in how many prescriptions are filled with generics - over 90%. That sounds great. But here’s the twist: Americans pay more for those generics than almost anyone else.
In 2022, U.S. drug prices - including both brand and generic - were 2.78 times higher than the average in other rich countries. Why? Because competition doesn’t always mean lower prices. In some cases, when only one company makes a generic, they raise prices sharply. A 2023 FDA report found 68% of the 147 drug shortages that year came from quality problems at single-source factories, mostly in India and China. When one plant shuts down, the whole market can freeze.
And it’s not just about supply. The U.S. doesn’t negotiate drug prices. Pharmacies and insurers pay what’s listed. So even if a pill costs $0.10 to make, you might pay $10. And if you travel to Canada or Mexico, you might find the exact same pill for $1.
Europe: A Patchwork of Rules
Europe doesn’t have one system. It has 27 different ones.
In the U.K., 83% of prescriptions are for generics. The government pushes substitution - pharmacists can swap a brand for a generic unless the doctor says no. Patients are used to it. Prices are low. A month’s supply of metformin might cost less than £2.
But in Switzerland? Only 17% of prescriptions are generics. Doctors and patients still trust the original brand. And the government pays more for it. Same drug. Same manufacturer. Same factory. But in Switzerland, it costs over six times what it does in the U.K.
Germany and the Netherlands sit in the middle - around 70-80% generic use. But even there, rules vary. In Germany, pharmacists must offer generics. In Italy and Greece, less than 20% of prescriptions are generic. Why? Lack of policy. Lack of trust. Lack of incentives.
And here’s another layer: regulatory delays. The European Medicines Agency approves a generic, but then each country has to approve it again. That adds 18 to 24 months before the drug hits shelves. That’s extra time the brand-name drug can keep charging high prices.

India: The Pharmacy of the World
India makes about 20% of all generic drugs used globally. Forty percent of the generics in the U.S. come from India. Over 750 Indian factories are approved by the FDA. That’s more than any other country.
But that doesn’t mean every Indian-made pill is the same. A 2023 study from Ohio State University found Indian-made generics had 54% higher rates of severe side effects - hospitalizations, disability, even death - compared to identical pills made in the U.S. That’s not because the active ingredient is different. It’s because of the fillers, the coating, the way it’s made. Small changes. Big consequences.
Patients on Reddit and pharmacy forums report switching from a U.S.-made generic to an Indian version and noticing new side effects - dizziness, nausea, fatigue. Doctors call it “therapeutic substitution shock.” The drug is technically the same. But the body reacts differently.
India’s market is growing fast - expected to hit $51 billion by 2033. But quality control is uneven. Some factories are world-class. Others cut corners to survive in a race to the bottom.
Why Do Prices Vary So Much?
It’s not about cost. It’s about control.
- Government pricing rules: In the U.K., the government sets maximum prices. In the U.S., no one does.
- Insurance and reimbursement: If insurers pay more for brands, doctors prescribe them. If they pay more for generics, doctors switch.
- Public trust: In Japan and Switzerland, patients believe brand-name = better. In the U.K. and Canada, they believe generics = smart.
- Manufacturing location: Drugs made in the U.S. or EU cost more to produce. Drugs made in India or China cost less - but carry higher risk of quality issues.
- Parallel trade: Some pharmacies in Germany buy cheap generics from Poland and resell them at a profit. That’s legal. But it creates shortages where the drugs are made.
One example: levothyroxine. Used by millions for thyroid problems. In the U.S., it’s made by several companies. In France, it’s mostly one brand. In India, it’s made by dozens. The active ingredient is identical. But the side effects? Not always.
What Happens When a Generic Is Delayed?
When a patent expires, the first generic maker usually gets a 180-day exclusivity period in the U.S. That means no other company can enter. So they jack up the price. Then, when others finally come in, the price crashes.
But sometimes, the first company never launches. They sit on the approval and block others. That’s called “evergreening.” Drugmakers file tiny patent changes - a new coating, a different shape - to delay generics. Between 2015 and 2022, over 1,200 such patents were filed on just 12 top-selling drugs.
That’s why some life-saving drugs - like insulin, asthma inhalers, or blood thinners - still cost hundreds of dollars even after 20 years. The system is broken.

What Can You Do?
If you’re taking a generic drug:
- Know your manufacturer. Check the label. If it changes, ask your pharmacist why.
- Don’t assume all generics are the same. If you feel different after switching, tell your doctor.
- Compare prices. Use tools like GoodRx. You might find the same pill cheaper in Canada or Mexico.
- Ask if your insurance covers a specific generic. Sometimes they push one brand over another.
If you’re traveling, bring your prescription and the name of your manufacturer. A generic you’ve been taking for years in the U.S. might not be available abroad - or might be made by a different company with different effects.
The Future: Will This Change?
There are signs of progress.
The WHO is pushing for global quality standards. The FDA is getting more funding to inspect foreign factories - and doing more unannounced checks. The U.S. Inflation Reduction Act now speeds up generic reviews by 30%. The EU wants 80% generic use by 2030.
But the biggest barrier isn’t science. It’s politics. Countries protect their own systems. Manufacturers protect their profits. Patients are caught in the middle.
AI might help. By 2030, machine learning could cut generic development time from five years to two. That could mean more competition, faster. But only if regulators keep up.
For now, the truth is simple: your medicine’s price has little to do with what it costs to make. It has everything to do with where you live, who controls the rules, and whether your system values your health - or its profits.
Why do generic drugs cost so much more in the U.S. than in other countries?
The U.S. doesn’t regulate drug prices. Pharmacies and insurers pay list prices, even for generics. In countries like the U.K. or Canada, the government sets price caps. In the U.S., if one company makes a generic and others don’t enter the market, that company can raise prices dramatically. High demand and low competition on certain drugs create price spikes - even when manufacturing costs are low.
Are generic drugs from India safe to take?
Many Indian-made generics are safe and FDA-approved. But not all are equal. A 2023 study found Indian-made generics had 54% higher rates of severe side effects compared to U.S.-made versions of the same drug. This isn’t because the active ingredient is different - it’s due to variations in inactive ingredients, manufacturing quality, or storage. Always check the manufacturer. If you notice new side effects after switching to an Indian generic, talk to your doctor.
Can I legally buy generic drugs from other countries?
Technically, importing prescription drugs into the U.S. is illegal, but the FDA rarely enforces this for personal use - especially if it’s a generic you already take. Many Americans buy from Canadian or Indian online pharmacies and save 60-80%. But there’s risk: counterfeit drugs, expired products, or poor quality control. Only use verified pharmacies like those listed on PharmacyChecker.com.
Why do some doctors refuse to prescribe generics?
Some doctors believe brand-name drugs are more reliable, especially for conditions like epilepsy, thyroid disease, or blood thinners, where small differences in absorption matter. Others are influenced by pharmaceutical reps or patient preference. In countries like Switzerland and Japan, patients expect brand names and will refuse generics. In the U.K., doctors are encouraged to prescribe generics to save money.
How do I know if my generic drug changed manufacturers?
Check the pill’s imprint code and color - they change with the manufacturer. Look at the pharmacy label: it should list the manufacturer’s name. If you notice new side effects, a different pill shape, or a sudden price change, ask your pharmacist if the generic was switched. Don’t assume it’s the same.
What’s the difference between a generic and a biosimilar?
Generics are exact chemical copies of small-molecule drugs (like metformin or atorvastatin). Biosimilars are copies of complex biological drugs (like insulin or Humira). They’re not identical - they’re very similar. Biosimilars are harder to make, more expensive, and take longer to get approved. They’re also less likely to be substituted automatically by pharmacists.
Why do some countries have more generic options than others?
It depends on how fast regulators approve generics, how much competition they allow, and whether they require pharmacies to substitute. The U.S. allows many manufacturers and has fast approval. Switzerland limits competition and pays more for brands. India produces generics in bulk but doesn’t regulate pricing. The number of manufacturers per drug ranges from 23% in Norway to 66% in the U.S.
What’s Next?
If you’re taking a generic drug, stay informed. Know your manufacturer. Track your symptoms. Compare prices. Ask questions. The system isn’t fair. But you don’t have to be powerless.
Generic drugs were meant to save lives - not just money. When the same pill costs $1 in one country and $100 in another, it’s not a market failure. It’s a moral one.

8 Comments
This is wild. I had a generic thyroid med from India last year-switched from the U.S. version-and suddenly I felt like I’d been hit by a truck. Dizziness. Nausea. Like my brain was slowly dissolving. I thought I was dying. Turns out, the fillers? Totally different. Same active ingredient? Sure. But your body isn’t a lab rat. It notices everything. And no one tells you this until you’re already on the floor.
And don’t get me started on the FDA inspections. They show up like a ghost. One day, factory’s clean. Next day? Shut down. Meanwhile, your prescription is empty. And the pharmacy? ‘Sorry, we’re out.’ Like it’s your fault you need to live.
Meanwhile, my cousin in Canada buys the same pill for $1.50. I pay $47. And the government says ‘market forces.’ Market forces my ass. This isn’t capitalism. It’s corporate extortion with a prescription pad.
Let me tell you what’s REALLY going on-because no one wants to say it out loud. The FDA, Big Pharma, and the WHO? All in bed together. The ‘quality control’ claims? A smokescreen. The real reason U.S. generics cost more? Because they’re deliberately made with inferior fillers to keep people dependent on brand-name drugs-so the patents can be extended under the guise of ‘safety.’
Did you know the FDA’s own data shows that 87% of drug shortages are caused by ONE manufacturer? That’s not coincidence. That’s strategy. They let one company dominate, then jack up the price. Then, when people complain? ‘Oh, we’re inspecting!’ Like that’s a solution. Meanwhile, Indian factories are producing FDA-approved pills that work better than U.S. versions-except they’re banned because they’re TOO cheap. That’s not regulation. That’s price-fixing with a lab coat.
And don’t even get me started on parallel trade. Germany buys generics from Poland, resells them, and creates shortages? That’s not free market. That’s economic warfare. The U.S. government knows this. They just don’t care. Because they’re getting kickbacks. From who? You figure it out.
And now they want to use AI to ‘speed up’ generics? Please. AI won’t fix corruption. It’ll just make it faster. And more invisible.
Wake up. This isn’t about medicine. It’s about control. And they’re using your body as collateral.
While I appreciate the emotional intensity of the previous comments, let’s ground this in data. The FDA approves over 750 Indian manufacturing sites-more than any other country. That’s not an accident; it’s a result of rigorous, unannounced inspections. Yes, there are outliers-but the 54% side effect statistic cited? It’s misleading. The study compared *self-reported* adverse events from Reddit forums to FDA adverse event reports-two entirely different data streams.
Also, the U.S. doesn’t ‘negotiate’ prices because we don’t have a single-payer system. That’s a policy choice, not a conspiracy. Countries like the U.K. cap prices because they pay directly. We pay indirectly via insurers, who negotiate in private-often resulting in higher list prices to cover administrative overhead.
And yes, some doctors resist generics for valid reasons: narrow therapeutic index drugs like warfarin or levothyroxine require consistency. But that’s not fear-it’s clinical prudence.
So yes, the system is flawed. But the solutions aren’t ‘buy from Canada’ or ‘ban India.’ They’re: 1) enforce transparency in sourcing, 2) incentivize multi-source competition, 3) standardize regulatory alignment. Not outrage. Strategy.
Also, I’m not saying this to be cold. I’ve been on a generic statin for 8 years. It saved me $300/month. I’m just tired of the noise drowning out the fix.
Look-I’ve been a pharmacist for 22 years. I’ve seen generics come and go. I’ve watched patients cry because their $2 pill suddenly became $80. I’ve seen people switch brands and feel like their body betrayed them. It’s real.
But here’s the quiet truth: most people never notice the difference. The vast majority of generics? Perfectly safe. Perfectly effective. The horror stories? They exist-but they’re outliers. Like winning the lottery… but for side effects.
What matters is consistency. If your pill looks different? Ask why. If your symptoms change? Tell your doctor. Don’t panic. Don’t quit. Just pay attention.
And if you’re saving $100 a month by buying from Canada? Do it. The FDA won’t knock on your door. They’ve got bigger fish to fry.
Be smart. Not scared. You’re not powerless. You just need to know how to ask the right questions.
And hey-if you’re reading this, you’re already ahead of 90% of people who just swallow the pill and hope for the best. 👍
It is deeply offensive to suggest that Indian pharmaceutical manufacturing is inherently inferior. India produces over 60% of the world’s generic vaccines and 40% of U.S. generic drugs. Our factories meet or exceed international standards. The FDA’s own reports confirm this.
Western narratives of ‘quality risk’ are colonial relics-rooted in bias, not science. When your country charges $100 for a $0.10 pill, it is not because of manufacturing. It is because of greed. Our manufacturers operate with dignity, precision, and scale. Your system is broken-not ours.
And to those who import from India? You are not criminals. You are survivors. The global South does not owe the West its medicine at inflated prices. We produce it. We deserve to be respected for it.
Stop blaming the pharmacy. Start blaming the price tag.
India did not build its pharmaceutical empire by cutting corners. We built it by outworking the West-and outthinking its profit-driven madness.
Hey, I work in a pharmacy in Mumbai, and I’ve seen this from the other side. A lot of folks here buy the same pills their Western friends pay $50 for-just for 20 rupees. We don’t have the luxury of choosing. So we trust the generics. Most of the time, they work fine.
But yeah, sometimes the color changes, or the pill tastes weird. We tell people: ‘If you feel off, tell your doctor. Don’t suffer silently.’
It’s not about ‘good’ or ‘bad’-it’s about awareness. And honestly? I’m glad people in the U.S. are finally asking these questions. We’ve been yelling about this for years. Now maybe someone’s listening.
And hey-if you’re buying from us? Thank you. We’re just trying to help people stay alive. No drama. Just medicine.
So let me get this straight: the U.S. pays $50 for a pill that costs $0.10 to make, India makes it for $2, and Switzerland pays $120 because people think it’s ‘prestigious’? And we’re surprised this system is broken?
Meanwhile, in South Africa, I’ve seen people split pills in half just to afford them. Not because they’re cheap here. Because they’re UNAFFORDABLE.
So yeah. The real question isn’t ‘why are prices different?’
It’s: why are we okay with this?
Also, I once bought a generic from a Canadian pharmacy. It was the same pill. Same box. Same imprint. But cheaper. I didn’t die. I saved $200. So… yeah. I’m not sorry.
Just wanted to say-this post made me feel less alone. I switched generics last year and had a panic attack thinking I’d been poisoned. Turns out, it was just a different filler. Took me three weeks to get my energy back.
Now I keep a little notebook: pill color, shape, manufacturer. I screenshot the label every time I refill. It’s weird, I know. But it’s my insurance policy.
And I use GoodRx like it’s my religion. Found a $3 version of my blood pressure med last month. Same pill. Different factory. No side effects.
You’re not crazy for noticing the difference. You’re smart. And you deserve to know what’s in your body.
Keep asking questions. Keep checking labels. And if you ever feel weird after a switch? Text your doctor. Don’t wait. You’ve got this. 💪