When a pharmacist fills a prescription for a medication like warfarin, levothyroxine, or phenytoin, they don’t just grab the cheapest generic off the shelf. In many states, they’re legally required to stop and think - because these are NTI drugs. Narrow Therapeutic Index drugs are a small but critical group of medications where even tiny changes in dose or blood levels can lead to serious harm - think blood clots, seizures, or even death. While the federal government says generic versions are just as safe, 27 states have passed laws that say otherwise. And those laws? They’re wildly different.
What Makes a Drug an NTI Drug?
NTI stands for Narrow Therapeutic Index. It’s not a fancy term - it just means the difference between a dose that works and a dose that’s dangerous is very small. Take warfarin, for example. A 5% change in blood concentration can turn a life-saving anticoagulant into a bleeding risk. Or levothyroxine, used for hypothyroidism. A slight shift in absorption can send thyroid-stimulating hormone (TSH) levels soaring or crashing, causing fatigue, weight gain, or heart problems. These aren’t theoretical risks. A 2023 meta-analysis of 17 studies found that over one-third of patients stabilized on brand-name levothyroxine needed dose adjustments after switching to a generic version.
The FDA doesn’t officially label any drugs as NTI. Their Orange Book, which rates drugs for therapeutic equivalence, only uses an "A" or "B" system. "A" means bioequivalent - meaning the generic absorbs into the body similarly to the brand. But here’s the catch: the FDA allows a 20% variation in absorption for generics. For most drugs, that’s fine. For NTI drugs? That’s like letting a pilot fly a plane with a gas gauge that’s off by a quarter tank.
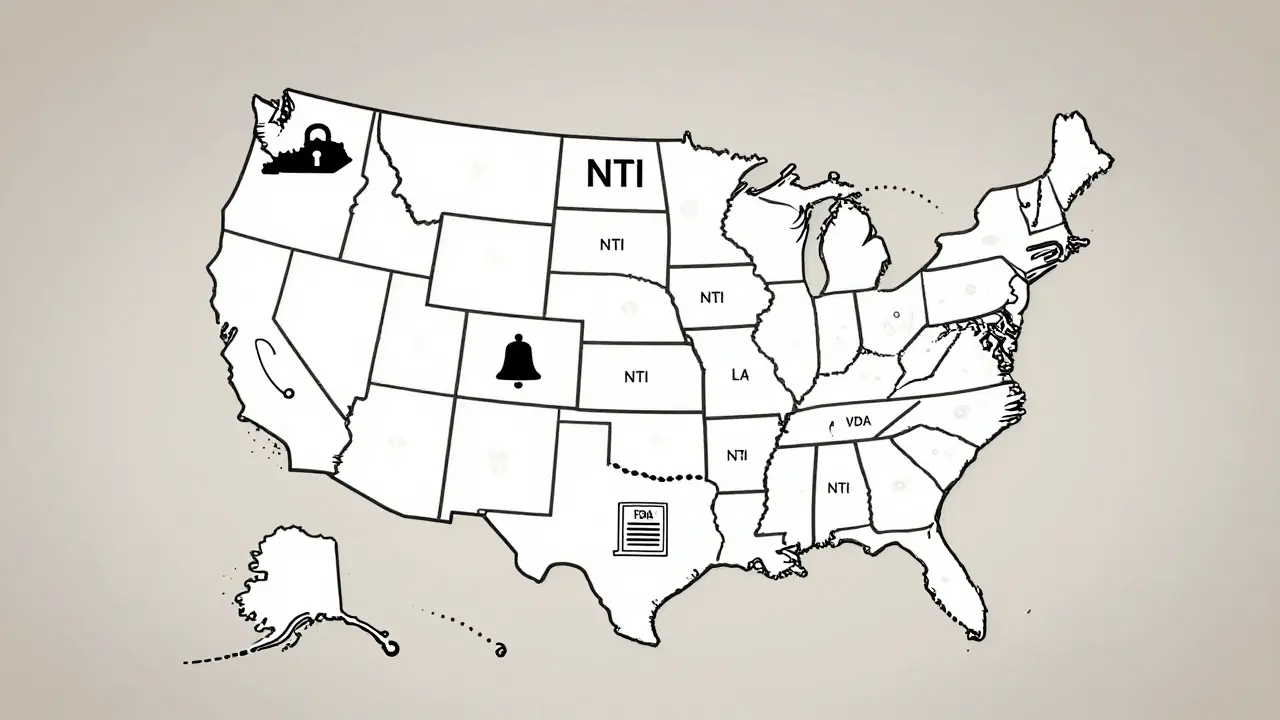
State Laws Are All Over the Map
While the FDA takes a hands-off approach, states have stepped in - and they’re not all doing the same thing.
In Kentucky and Pennsylvania, pharmacists can’t substitute generics for certain NTI drugs without explicit permission from the prescriber. Their lists are specific: digitalis glycosides, antiepileptics, warfarin, and lithium are locked down. No exceptions.
South Carolina doesn’t ban substitution outright. Instead, it recommends against it for three categories: NTI drugs, brand-specific meds like Synthroid and Premarin, and "critical drugs" including insulin, anticoagulants, and time-release asthma inhalers. It’s advisory, not law - but most pharmacists treat it like a rule.
Tennessee lets pharmacists substitute A-rated generics - unless the patient has epilepsy or seizures. Then, no substitution. Period. That’s a targeted exception, not a blanket ban.
California goes further. Its law defines "critical dose drugs" as those where a 10% or less change in blood concentration could be dangerous. Pharmacists must notify the prescriber anytime they substitute one. That’s not just a warning - it’s a legal requirement.
And then there’s Iowa. They don’t make their own list. They tell pharmacists to just use the FDA’s Orange Book. No extra rules. No special lists. Simple.
Why the Chaos?
The inconsistency isn’t random. It’s a reaction to real patient harm. A 2022 study in the Journal of the American Pharmacists Association found that states with NTI substitution restrictions saw 18.7% fewer adverse events related to warfarin. That sounds big - until you realize it was only a 0.3% drop in total events. Still, for the patients who had a bleed or clot because of a switch? That 0.3% matters.
But the flip side? Pharmacy chaos. A 2023 survey by the National Community Pharmacists Association found that nearly 70% of pharmacists who work across state lines get confused by the rules. Over 40% admitted they accidentally broke substitution laws in the past year. One pharmacist in Tennessee described it like this: "In Knoxville, I can’t switch antiepileptics. In Chattanooga - same state - I have to check if the patient’s doctor has a different policy. It’s a mess."
Who’s Right? FDA or the States?
The FDA says the current bioequivalence standards are enough. Dr. John Jenkins, former head of the FDA’s drug evaluation office, called state restrictions "unnecessary." He argues that if a generic passes the 20% bioequivalence test, it’s safe.
But doctors like Dr. Jerry Avorn from Harvard say that’s dangerously naive. "For levothyroxine," he wrote in JAMA Internal Medicine, "a 5-10% variation can destabilize a patient who’s been stable for years." The American College of Clinical Pharmacy agrees. Their 2023 position statement backs state-level restrictions, citing evidence that switching generics often triggers thyroid level swings.
The FDA did create an NTI list in 1995 - but never made it official. That contradiction fuels the debate. If the FDA doesn’t classify these drugs, why do states? Because they’ve seen what happens when a patient’s dose changes without warning.
What’s Changing in 2025?
Things are starting to shift. In January 2024, the National Association of Boards of Pharmacy released a Model State NTI Substitution Act. It proposes a single, evidence-based list of NTI drugs - no more 50 different lists. Twelve states have already introduced it as legislation.
Meanwhile, the FDA is reconsidering its stance. In September 2024, they announced they’d review their position after the Senate Committee on Aging pointed to a Government Accountability Office report: 2,847 adverse events linked to NTI drug substitutions between 2019 and 2023. That’s not a small number.
And it’s not just about generics. Biosimilar substitution rules are now in place in 48 states. Pharmacists are juggling two sets of confusing rules - one for small-molecule NTI drugs, another for biologics. It’s a regulatory tangle that’s costing pharmacies money. Express Scripts reported a 5.7% rise in administrative costs just from NTI-related restrictions.

What This Means for Patients
If you take warfarin, levothyroxine, or an antiepileptic - check your prescription label. Does it say "dispense as written"? If not, your pharmacist might switch you without telling you. In restrictive states, they can’t. In others, they can - and you might not know.
Ask your pharmacist: "Is this drug on my state’s NTI list?" If they hesitate, ask your doctor to write "do not substitute" on the prescription. It’s not a hassle - it’s a safety step.
And if you’ve ever had your thyroid levels go haywire after a generic switch, you’re not alone. You’re part of a growing group pushing for change - and that change is finally starting to move.
What’s Next?
By 2027, IQVIA predicts 38 states will adopt standardized NTI substitution rules. That means fewer errors, fewer hospital visits, and less confusion for pharmacists. But it also means fewer generic switches - which could mean higher costs for patients and insurers.
The bottom line? NTI drugs aren’t just another prescription. They’re precision medicine. And when the margin for error is this thin, one-size-fits-all doesn’t cut it.