When you pick up a prescription for something like lisinopril or metformin, there’s a good chance it’s not the brand name you remember from TV ads. It’s a generic drug - cheaper, just as effective, and made through a process most people never think about. But how exactly are these life-saving medications produced? And why can they cost up to 85% less than the original brand? The answer lies in a tightly controlled, highly scientific process that balances speed, cost, and safety - all under the watchful eye of regulators like the FDA.
Starting with the Original: Reverse Engineering the Brand-Name Drug
The journey of a generic drug doesn’t begin with a lab coat and a beaker. It starts with the brand-name version already on the market. Manufacturers don’t invent new drugs - they copy them. But copying isn’t as simple as copying a recipe. They have to reverse engineer the original product down to the last detail. This means identifying the exact active pharmaceutical ingredient (API) - the molecule that actually treats the condition - and every single excipient (the inactive ingredients like fillers, binders, and coatings). A tablet of a brand-name drug might contain lactose, magnesium stearate, and hypromellose. The generic version must use the same ones, or prove that a substitute won’t change how the drug works. This step is called analyzing the Reference Listed Drug (RLD). It’s not just about matching ingredients. It’s about understanding how they interact. For example, the particle size of the API, the way it’s granulated, even the humidity during production - all these affect how fast the drug dissolves in your body. If the generic dissolves too slowly, you won’t get enough medicine into your bloodstream. Too fast, and you could get too much at once. That’s why the FDA requires exact matching of Critical Quality Attributes (CQAs) - the measurable traits that determine safety and effectiveness.Designing the Formula: Quality by Design (QbD)
Once the RLD is fully understood, manufacturers move into formulation development using the Quality by Design (QbD) framework. This isn’t trial and error. It’s a science-based approach rooted in the International Council for Harmonisation (ICH) guidelines. Engineers identify three key factors:- Critical Quality Attributes (CQAs): What must the final product do? (e.g., dissolve in 30 minutes, release 95% of API within 45 minutes)
- Critical Material Attributes (CMAs): What properties of the raw materials matter? (e.g., purity of API, moisture content of lactose)
- Critical Process Parameters (CPPs): What steps in production must be tightly controlled? (e.g., mixing time, compression pressure, drying temperature)
The Seven-Step Manufacturing Process
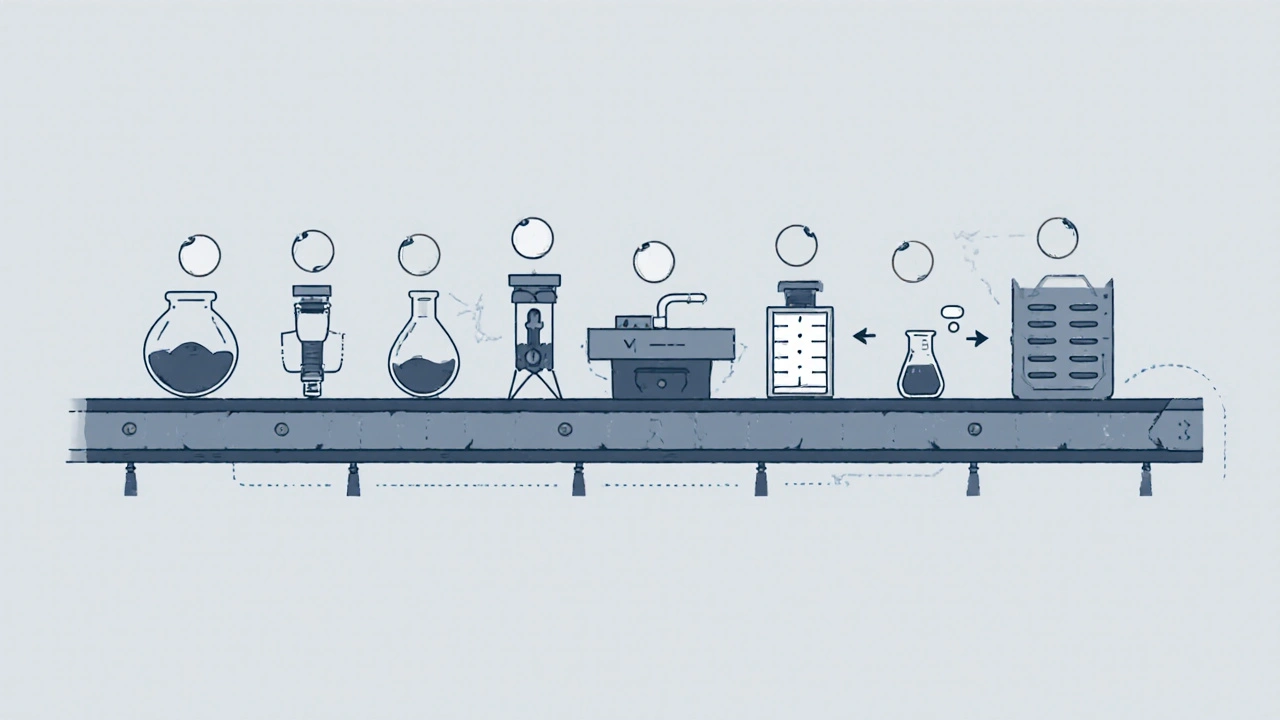
Once the formula is locked in, production begins. Here’s what happens in a typical generic drug manufacturing facility:- Formulation: The API and excipients are weighed with extreme precision - often to the milligram. Even a 1% error can throw off the entire batch.
- Mixing and Granulation: Ingredients are blended in large tumblers or fluid bed mixers. For tablets, they’re often turned into granules - small clumps that flow better during compression. This step ensures every tablet has the exact same amount of active ingredient.
- Drying: If the granules are wet from the mixing process, they’re dried in ovens or fluid bed dryers. Moisture can cause the drug to degrade or stick to machinery.
- Compression and Encapsulation: Dry granules are pressed into tablets using high-speed tablet presses that can make 500,000 tablets per hour. Capsules are filled using automated machines that drop precise powder amounts into gelatin shells.
- Coating: Tablets get a thin film coating - not just to make them look nicer, but to protect the drug from light, moisture, or stomach acid. Some coatings are designed to delay release, like extended-release versions of metformin.
- Quality Control: This isn’t just a final check. Testing happens at every stage. Tablets are checked for weight variation (±5% for tablets under 130mg), hardness, thickness, and dissolution rate. A sample from each batch is dissolved in a simulated stomach fluid to confirm it releases the API at the same rate as the brand-name version.
- Packaging and Labeling: Bottles or blister packs are filled, sealed, and labeled. The label must match the brand-name drug’s information - same dosage, same warnings, same instructions. But the color and shape? Those can be different. U.S. law forbids generics from looking identical to brand-name drugs to avoid confusion.
Regulatory Hurdles: The ANDA Pathway
You can’t just make a generic and sell it. You have to get approval from the FDA through the Abbreviated New Drug Application (ANDA). This is where the process gets its name - “abbreviated” because you don’t need to repeat the 10-year clinical trials the brand-name company did. Instead, you prove bioequivalence. That means your drug is absorbed into the bloodstream at the same rate and to the same extent as the original. This is tested in 24-36 healthy volunteers. Blood samples are taken over 24-72 hours to measure two key numbers:- Cmax: The highest concentration of the drug in the blood
- AUC: The total amount of drug absorbed over time
Where Things Go Wrong: Common Manufacturing Challenges
Even with strict rules, things can go wrong. One of the biggest headaches for manufacturers is excipient variability. A batch of lactose from one supplier might have slightly different particle sizes than the last. That tiny change can alter how the tablet compresses or dissolves. One pharmaceutical engineer on Reddit said it’s like baking bread with flour from two different brands - same name, different results. Another issue is process validation. The FDA requires proof that every step in manufacturing consistently produces the same result. If a tablet press starts making pills that are too soft, the company must investigate within 24 hours, fix the problem, and document everything. Failure to do so can lead to warning letters or recalls. In 2021, Teva recalled 14 generic products after FDA inspectors found serious CGMP violations at its Puerto Rico plant. Problems included unclean equipment, unverified test results, and poor documentation. That’s why the FDA inspects generic drug facilities more than 1,000 times a year - more than any other type of drug manufacturer.Complex Generics: The New Frontier
Not all generics are created equal. Simple pills like ibuprofen or amoxicillin are easy to copy. But complex products - like inhalers, nasal sprays, eye drops, or long-acting injections - are much harder. Why? Because how the drug is delivered matters as much as what’s inside. Take an asthma inhaler. It’s not just about the active ingredient. It’s about the propellant, the valve design, the spray pattern, and how fine the mist is. A generic version might have the same chemical, but if the spray doesn’t reach deep into the lungs, it won’t work. The FDA has published 127 product-specific guidances for these complex generics since 2020. One case study showed a company spending seven years and $47 million to match a topical steroid cream. They had to develop new testing methods just to prove their version penetrated the skin the same way.
Why It Matters: Cost, Access, and Trust
In 2023, 90% of all prescriptions filled in the U.S. were for generic drugs. That’s over $1.7 trillion saved in a decade. Without generics, millions of people couldn’t afford their medications. A heart drug like lisinopril costs $4 a month as a generic. The brand version? Over $100. But trust is fragile. A 2022 study in JAMA Internal Medicine raised concerns about variability in multi-source generics - especially for drugs with a narrow therapeutic index, like warfarin or levothyroxine. One patient might get a tablet from one manufacturer that works perfectly, then switch to another and feel off. That’s why pharmacists often stick to one generic brand for these drugs. Still, a 2023 survey found 89% of pharmacists have high confidence in generic quality. And for most people, generics work just as well - every day, in every pharmacy, across the country.What’s Next: AI, Continuous Manufacturing, and Global Supply Chains
The industry is changing. The FDA has approved 17 facilities using continuous manufacturing - a system where ingredients flow through machines nonstop, like a conveyor belt. This reduces production time from weeks to hours and cuts errors. One company using this method for a cystic fibrosis drug now has a 99.98% batch acceptance rate. Artificial intelligence is also being tested. Pfizer’s pilot program used AI to scan tablets for defects - reducing visual inspection errors by 40%. Digital twins - virtual models of manufacturing lines - are being used to predict problems before they happen. But there’s a big concern: supply chains. Nearly 80% of the active ingredients in U.S. generic drugs come from China and India. A single factory shutdown or regulatory issue overseas can cause nationwide shortages. That’s why the FDA is pushing for more domestic production and better tracking of raw materials.Final Thoughts: Safe, Effective, and Essential
Generic drugs aren’t cheap because they’re low quality. They’re cheap because the system is designed to eliminate unnecessary costs - not cut corners. Every step, from the API to the label, is scrutinized. Every batch is tested. Every facility is inspected. The process is complex, demanding, and highly regulated. And it works. For most people, taking a generic drug is no different than taking the brand name - same effect, same safety, same outcome. The only difference? The price tag.Are generic drugs as safe and effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they’re absorbed into the body at the same rate and to the same extent. Over 90% of prescriptions in the U.S. are filled with generics, and decades of real-world use confirm they work just as well.
Why do generic drugs look different from brand-name drugs?
U.S. trademark laws prevent generic drugs from looking identical to brand-name versions. That means the color, shape, size, or flavor can be different - but the active ingredient and how it works are exactly the same. These differences are purely cosmetic and don’t affect safety or effectiveness.
Can different generic brands of the same drug work differently?
For most drugs, no. All generics must meet the same FDA standards. But for drugs with a narrow therapeutic index - like warfarin, levothyroxine, or some seizure medications - even small differences in how the drug is absorbed can matter. Some patients and doctors prefer to stick with one generic manufacturer for consistency. If you notice a change in how you feel after switching generics, talk to your pharmacist or doctor.
How long does it take to make a generic drug?
It typically takes 3-4 years and $5-10 million to develop and get approval for a generic drug. This includes reverse engineering the original, developing the formula, running bioequivalence studies, and passing FDA inspections. Simple pills are faster; complex products like inhalers or topical creams can take 7+ years.
Are generic drugs made in the same kind of facilities as brand-name drugs?
Yes. Generic drug manufacturers must follow the same Current Good Manufacturing Practices (CGMP) as brand-name companies. The FDA inspects both types of facilities using the same standards. Many generic manufacturers even supply ingredients or finished products to brand-name companies under contract.
Why are some generic drugs cheaper than others?
Price differences come down to competition and production scale. When multiple companies make the same generic, prices drop quickly - sometimes by 80% within two years. But if only one or two manufacturers are producing it, prices stay higher. Complex generics with fewer competitors also cost more to make, so they’re priced higher. Market dynamics, not quality, drive the price differences.





8 Comments
Just had to say this: I’ve been on generic lisinopril for 5 years and my BP is rock solid. No side effects, no drama. My pharmacy charges me $3 for a 30-day supply. Meanwhile, my cousin in Canada pays $120 for the brand. The system works - if you let it.
Let’s be real - if generics were truly equivalent, why does the FDA need 10,000 pages of documentation just to approve one? They’re not the same. They’re legally permitted approximations. And don’t even get me started on the 80-125% bioequivalence window. That’s not science - that’s a loophole dressed up as regulation.
80% of our meds come from China and India? Yeah, and you know what else comes from there? fake AirPods, toxic toothpaste, and that ‘organic’ turmeric that’s laced with lead. The FDA inspects ‘em? Sure, right after they get a tip-off. Meanwhile, my grandma’s levothyroxine switched brands and she started having panic attacks. Coincidence? Nah. It’s corporate greed with a lab coat.
It’s funny how we’ve turned medicine into a math problem - dissolve rate, Cmax, AUC - as if the human body is just a beaker in a factory. We’ve forgotten that pills aren’t widgets. They’re molecules that interact with souls, not just enzymes. Maybe the real question isn’t whether generics work… but whether we’ve stopped caring enough to notice when they don’t.
Bro, I work in a pharma lab in Hyderabad - we churn out 2 million tablets a day. The QA team is insane. Every batch gets tested for dissolution, hardness, purity - like, we run HPLC on 10 samples per batch. One time, a batch failed because the lactose had 0.3% more moisture. We tossed 500k pills. No one got rich doing this. But we don’t cut corners - because if we did, someone’s kid might not get better. Real talk.
Let’s not pretend this isn’t a foreign dependency crisis. We outsource the heart of our healthcare to two countries that don’t even speak our language. And now we’re surprised when there’s a shortage? Wake up. This isn’t capitalism - it’s national suicide with a pill bottle.
While the preceding comments reflect a spectrum of emotional and ideological perspectives, it is empirically verifiable that the Current Good Manufacturing Practices (CGMP) regulatory framework, as codified under 21 CFR Parts 210 and 211, applies uniformly across all pharmaceutical manufacturing entities, irrespective of brand or generic designation. The FDA’s inspectional authority and enforcement mechanisms are neither selective nor discretionary. To suggest otherwise is to misrepresent the statutory foundation of pharmaceutical regulation in the United States.
The entire paradigm of bioequivalence is a postmodern epistemological collapse - a performative simulacrum of therapeutic equivalence. We’ve replaced ontological certainty with statistical probability. Cmax and AUC aren’t measures of biological fidelity - they’re algorithmic proxies for market efficiency. The patient becomes a data point in a supply chain optimization model. And we call this progress? The body isn’t a variable in a regression. It’s a phenomenological experience. And yet, we’ve outsourced its integrity to a spreadsheet.