When a child gets pneumonia or an adult develops a urinary tract infection, the expectation is simple: a few days of antibiotics, and they’ll feel better. But for millions around the world, that’s no longer a guarantee. Antibiotic shortages are no longer rare exceptions-they’re a routine crisis. In 2024, the U.S. FDA tracked 147 active antibiotic shortages. Across Europe, 14 countries called their shortages “critical.” In low-income regions, patients are being sent home without treatment because the basic drugs simply aren’t there. This isn’t a glitch in the system. It’s a breakdown.
Why Antibiotics Are More Likely to Vanish Than Other Drugs
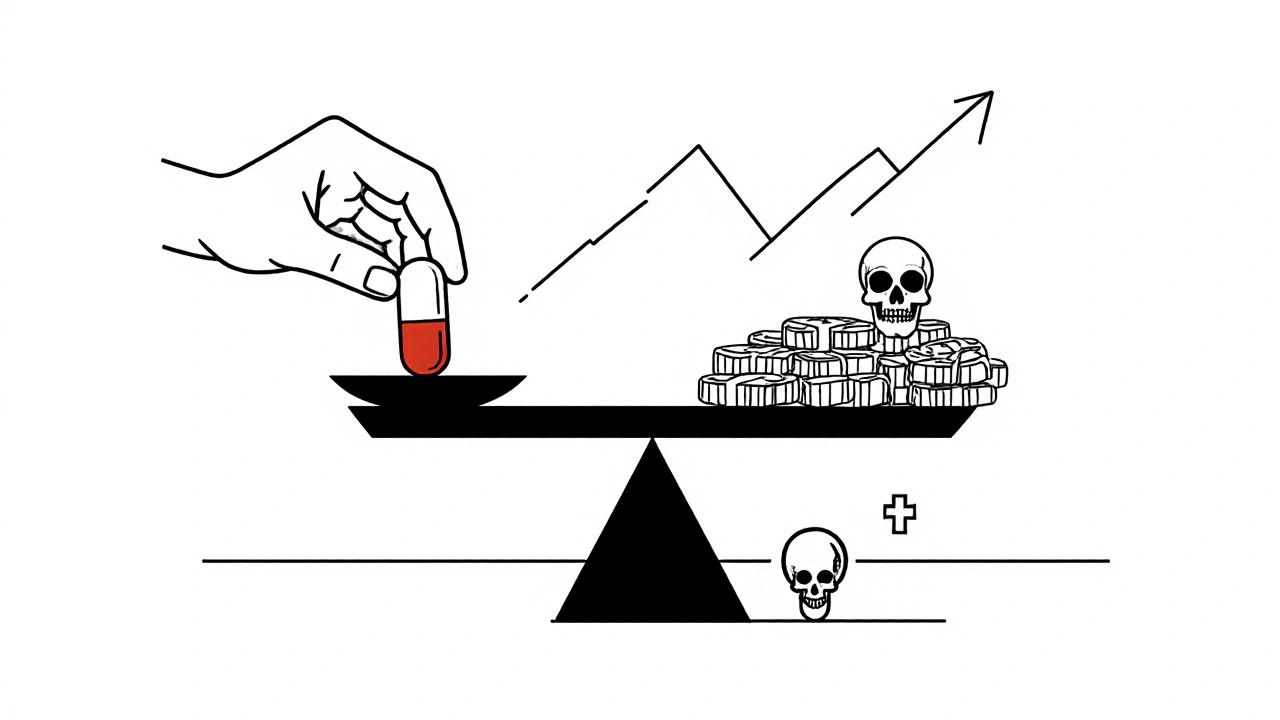
Antibiotics are 42% more likely to go into shortage than any other type of medication. Why? It’s not because they’re hard to make. It’s because no one wants to make them. Generic antibiotics-like penicillin, amoxicillin, and ciprofloxacin-cost pennies. A single dose of amoxicillin might sell for less than 25 cents. But producing it requires sterile facilities, strict quality checks, and trained workers. Those costs have gone up 34% since 2015. Meanwhile, the price of the drug itself has dropped 27% over the same period. Manufacturers are caught between rising expenses and shrinking profits. So they stop making them.The result? Only 85% of antibiotics used globally are generics. And those are the ones in shortest supply. Big pharmaceutical companies have moved on to more profitable drugs-cancer treatments, diabetes meds, rare disease therapies. Antibiotics? They’re seen as commodities, not investments. But unlike a blood pressure pill, there’s no backup. If you run out of amoxicillin, you can’t just switch to another antibiotic that works just as well. Many bacteria are already resistant. The alternatives are often older, more toxic, or harder to get.
What Happens When the Antibiotics Run Out
When antibiotics disappear, doctors don’t just wait. They improvise-and patients pay the price.In California, an infectious disease specialist told the American Public Health Association she had to use colistin-a last-resort antibiotic known for severe kidney damage-to treat a simple UTI. Why? Because first-line drugs like ampicillin and cephalexin weren’t available. Colistin isn’t meant for routine cases. It’s a weapon of last resort. But when you have no other option, you use it. And every time you do, you push bacteria closer to total resistance.
In the UK, hospitals started rationing amoxicillin after Brexit disrupted supply chains. One doctor on Reddit described having to choose between giving a child a full course of antibiotics or splitting doses to stretch supplies. In Kenya, nurses report sending children home with pneumonia because penicillin isn’t in stock. In Mumbai, a mother waited 72 hours for azithromycin to treat her child’s pneumonia. By the time it arrived, the infection had worsened. The child ended up in intensive care.
These aren’t isolated stories. A 2025 survey found 78% of U.S. hospital pharmacists had to change treatment plans because of antibiotic shortages. Sixty-two percent said patients had more complications as a result. In Europe, when amoxicillin with clavulanate disappeared in early 2023, usage dropped by 69%. That didn’t mean fewer infections. It meant fewer treated infections.
The Global Divide: Rich Countries, Poor Outcomes
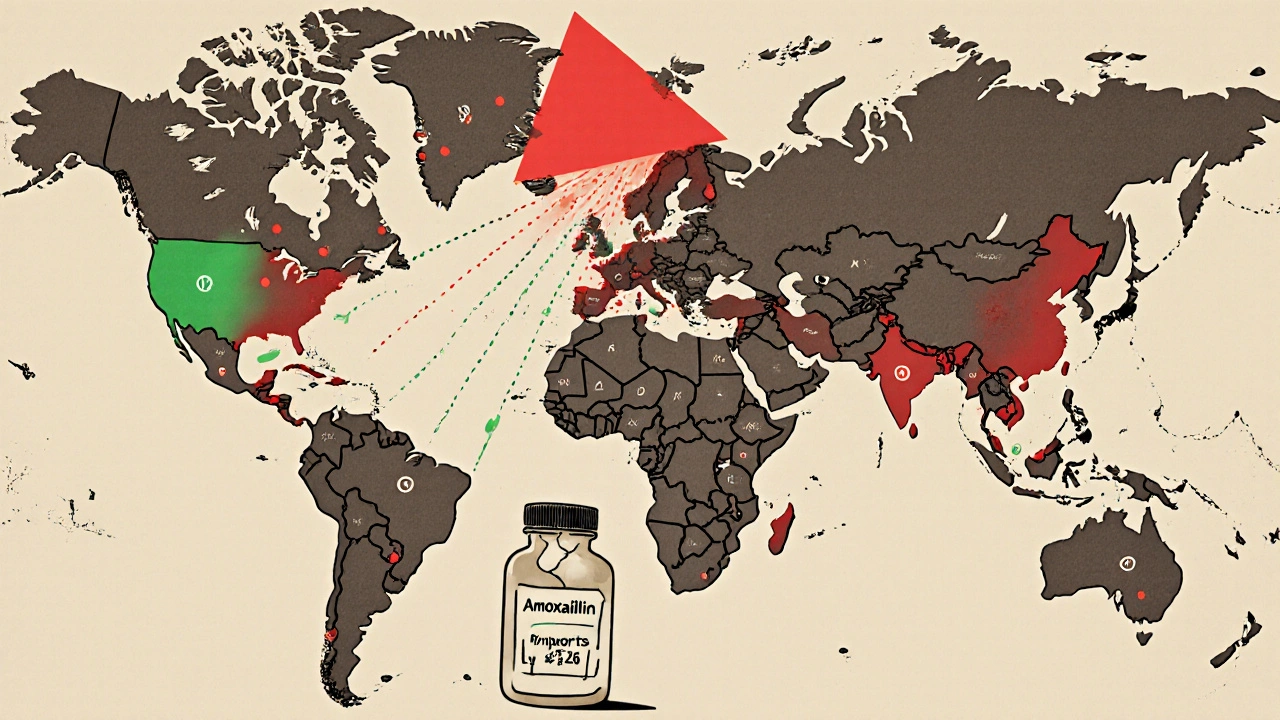
High-income countries can sometimes patch the gap. The U.S. imports antibiotics from India and China. Hospitals set up regional sharing networks. California’s network, launched in 2024, cut critical shortage impacts by 43% across 15 hospitals. But these are temporary fixes. They don’t solve the root problem.In low- and middle-income countries, there’s no safety net. Seventy percent of antibiotics are already inaccessible in these regions, according to WHO data. People don’t just face shortages-they face complete absence. A child with strep throat might never get penicillin. A woman with a postpartum infection might die because the hospital ran out of ceftriaxone. The WHO calls this a “syndemic”-a deadly mix of antibiotic resistance and lack of access. Where surveillance is weak, resistance grows unchecked. Where treatment is unavailable, infections turn deadly.
Even in places with strong health systems, the pressure is mounting. The European Court of Auditors found that regulatory agencies failed to enforce manufacturing standards. Facilities that produce sterile injectables-like penicillin G benzathine-require massive investment. But with prices so low, companies won’t spend the money. So production shuts down. And once it’s gone, restarting it takes years.
Resistance Is Rising-And Shortages Are Making It Worse
Antibiotic shortages don’t just mean untreated infections. They mean worse resistance.When a first-line antibiotic like ceftriaxone isn’t available, doctors turn to carbapenems. These are powerful, broad-spectrum drugs meant for the most serious infections. But overuse turns them into the new last resort. Globally, over 40% of E. coli and over 55% of K. pneumoniae are now resistant to third-generation cephalosporins. That means the drugs we once relied on for common infections no longer work.
Between 2018 and 2023, resistance rose in over 40% of the pathogen-antibiotic combinations tracked by WHO. That’s an annual increase of 5% to 15%. And it’s accelerating. In the WHO South-East Asian and Eastern Mediterranean regions, one in three infections is resistant. In Africa, it’s one in five. The World Health Organization now calls antimicrobial resistance a “global equity and health-systems crisis.”
It’s a vicious cycle: shortages force broader antibiotic use → broader use drives resistance → resistance makes more drugs useless → more drugs go into shortage. And the cycle keeps spinning.
What’s Being Done-And Why It’s Not Enough
There are efforts to fix this. The WHO announced a five-point plan in October 2025, including a $500 million Global Antibiotic Supply Security Initiative to be launched by 2027. The European Commission is rolling out its Pharmaceutical Strategy for Europe, targeting antibiotic shortages by 2026. The U.S. FDA approved two new manufacturing facilities in January 2025, expected to ease 15% of current shortages by late 2025.Hospitals are trying, too. Johns Hopkins implemented rapid diagnostic testing to cut unnecessary antibiotic use by 37% during shortages. Antimicrobial stewardship programs (ASPs) are now in 82% of U.S. hospitals-up from 50% in 2017. But only 37% of those programs meet all WHO standards. Most still lack the funding, staff, or tools to be truly effective.
The problem isn’t lack of awareness. It’s lack of investment. The global antibiotic market was worth $38.7 billion in 2024-but grew at just 1.2% per year since 2019. Meanwhile, the broader pharmaceutical industry grew at 5.7%. Investors aren’t betting on antibiotics. Why? Because they don’t make money. Even with new public-private partnerships promising 22% more R&D funding through 2027, manufacturing infrastructure is lagging. You can’t just invent your way out of this. You need factories. You need workers. You need stable prices.
What Patients and Providers Can Do Now
Waiting for governments or corporations to fix this could cost lives. But there are actions that can help right now.- Don’t demand antibiotics for viral infections. Colds, flu, and most sore throats don’t need antibiotics. Every unnecessary dose fuels resistance.
- Complete your full course. If you’re prescribed antibiotics, take them all-even if you feel better. Stopping early leaves behind the toughest bacteria.
- Support stewardship programs. Hospitals with strong ASPs reduce inappropriate use and stretch supplies. Ask if your hospital has one.
- Advocate for policy change. Push for government incentives to restart domestic antibiotic production. Support funding for global access programs.
For clinicians, the key is precision. Rapid diagnostics can tell you if an infection is bacterial or viral within hours. That means fewer broad-spectrum prescriptions. That means fewer shortages. That means fewer deaths.
The Future Is Bleak-Unless We Act
Without major intervention, global antibiotic shortages will increase by 40% by 2030, according to the Review on Antimicrobial Resistance. That could mean 1.2 million more deaths each year from infections we once treated easily. The WHO’s goal-to have 70% of antibiotic use come from the safer “Access” group by 2030-is currently at just 58%. We’re falling behind.This isn’t just a pharmacy problem. It’s a public health emergency. A national security threat. A moral failure. Antibiotics are one of the most important medical discoveries in history. They turned infections from death sentences into manageable conditions. But now, because of neglect, profit motives, and broken supply chains, we’re losing them.
If we don’t fix the system-by paying manufacturers fairly, investing in production, and protecting the drugs we have-we won’t just lose antibiotics. We’ll lose the ability to treat the most basic infections. And that’s a future no one should accept.
Why are antibiotics in short supply when we need them more than ever?
Antibiotics are mostly low-cost generics, so manufacturers earn little profit. Rising production costs-like regulatory compliance and sterile facility maintenance-have made them unprofitable. Companies have shifted to more lucrative drugs, leaving antibiotic production underfunded and unstable. This has led to factory closures and supply chain gaps, especially after events like Brexit.
What happens when a hospital runs out of penicillin or amoxicillin?
Doctors are forced to use alternatives, often broader-spectrum antibiotics like carbapenems or colistin. These drugs are more toxic, less effective for some infections, and accelerate antibiotic resistance. In low-resource areas, patients may get no treatment at all, leading to preventable deaths from simple infections like pneumonia or strep throat.
Are there any safe alternatives to antibiotics during a shortage?
There are no direct substitutes for antibiotics when treating bacterial infections. Antivirals, antifungals, or herbal remedies won’t work. The only alternatives are other antibiotics-which may be less effective, more toxic, or also in short supply. Prevention, rapid diagnostics, and strict stewardship are the best tools to reduce reliance on scarce drugs.
How are low-income countries affected by antibiotic shortages?
Severely. In many low-income countries, 70% of antibiotics are already inaccessible. Shortages mean patients go untreated for infections that were once curable. There’s no import system, no backup suppliers, and often no diagnostics to guide treatment. This leads to higher death rates and faster spread of resistant strains, creating a cycle of crisis.
Can we just make more antibiotics to fix this?
It’s not that simple. Building new manufacturing facilities takes years and billions in investment. Even with new U.S. plants expected to open in 2025, they’ll only cover 15% of current shortages. The bigger issue is economics: without guaranteed pricing and long-term contracts, companies won’t invest. Antibiotics aren’t profitable enough to justify the risk.
What can I do as a patient to help reduce antibiotic shortages?
Don’t pressure doctors for antibiotics if you have a virus. Always finish your full course if prescribed. Don’t save leftover antibiotics for later. Support policies that fund antibiotic production and global access. Every unnecessary use contributes to resistance and increases demand on a fragile supply.




9 Comments
It’s insane that we’re treating life-saving antibiotics like disposable commodities. I work in pediatrics, and I’ve had to explain to parents that we can’t fill their kid’s amoxicillin script-not because of supply chain issues, but because the manufacturer just stopped making it. These aren’t luxury drugs. They’re the baseline of modern medicine. We’re gambling with kids’ lives because Big Pharma decided it’s more profitable to sell a $10,000 cancer pill than a 25-cent antibiotic. This isn’t capitalism-it’s negligence.
Let’s be real-this is what happens when you outsource everything to China and India. We used to make penicillin in Ohio. Now we’re begging foreign factories to send us basic meds like they’re donating candy. If we want to stop this, we need to bring manufacturing back. Tariffs on foreign antibiotics. Tax breaks for U.S. antibiotic plants. National security isn’t just about tanks and drones-it’s about having enough amoxicillin to treat a 5-year-old with pneumonia. Wake up, America.
The economic disincentive structure for generic antibiotic production is fundamentally misaligned with public health imperatives. The marginal cost of production has increased significantly due to regulatory compliance and sterilization requirements, while price elasticity remains inelastic due to the absence of viable substitutes. Consequently, rational actors in the pharmaceutical sector have reallocated capital toward higher-margin therapeutic areas. This is not a failure of morality but of market design. Structural intervention-such as guaranteed minimum purchase agreements or public production models-is required to correct this externality.
Let me tell you something-this isn’t just about drugs. It’s about who we are as a society. We let a kid in Kenya die because we couldn’t be bothered to pay a few extra cents for a vial of penicillin. We let moms in Mumbai wait three days because Wall Street decided antibiotics were too boring to invest in. We’re not just failing patients-we’re failing our own humanity. And if you think this is just a ‘healthcare issue,’ you’re delusional. This is a war. And we’re losing. Time to stop being polite and start fighting like our lives depend on it-because they do.
I’ve seen this firsthand in rural England. A mother came in with her son, feverish, struggling to breathe. We had no amoxicillin. We had no alternatives. We gave her paracetamol and a referral to a hospital three counties away. She cried. I cried. And we both knew it shouldn’t have come to this. The system is broken-not because of greed alone, but because we stopped valuing the small things. Antibiotics are the quiet heroes of modern medicine. We took them for granted until they vanished. Now we’re scrambling. It’s too late to pretend we didn’t see this coming.
India makes 70% of the world’s generic antibiotics and you still complain? We have factories running 24/7 with quality control better than your FDA-approved labs. You Americans think you can fix this by building more plants? Ha! You don’t even know how to make a sterile vial. We’ve been doing this since the 1970s. Stop blaming others and fix your own broken pricing system. Also stop hoarding antibiotics like they’re gold. You think you’re smart taking leftover pills? You’re just breeding superbugs. Grow up.
It is important to note that the article does not sufficiently address the role of antibiotic overprescription by physicians as a primary driver of resistance. The focus on manufacturing and profit margins, while valid, ignores the behavioral component of the crisis. Furthermore, the suggestion that patients should 'advocate for policy change' is vague and lacks actionable guidance. The tone, while urgent, remains superficially performative.
Man, I just hope my kid never gets sick. I read this whole thing and I’m just sitting here thinking-what if the next time she gets an ear infection, there’s no amoxicillin? We’re one bad flu season away from a nightmare. I don’t know how to fix it, but I know we gotta stop acting like it’s someone else’s problem. Maybe start by not asking for antibiotics every time you have a sniffle. That’s something.
Just one thing: if you’re prescribed antibiotics, take them all. No saving them. No skipping doses. I’ve seen people do this and then get sicker later. It’s not just about resistance-it’s about your own health. And if your hospital runs out? Ask if they have an antimicrobial stewardship program. Most do now. They’re the unsung heroes keeping this from getting worse.