When you hear the name ranitidine, you probably think of heartburn medicine that disappeared from shelves a few years back. But what exactly was it, why did it vanish, and what should you do if you still need an H2 blocker? This guide breaks down the science, the safety drama, dosing basics, and the options you have today.
What is Ranitidine?
Ranitidine is a histamine H2‑receptor antagonist used to reduce stomach acid production. It belongs to the class of drugs called H2 blockers, which work by blocking the H2 receptors on stomach parietal cells, preventing histamine from triggering acid secretion.
How Ranitidine Works - The Simple Science
The stomach lining contains parietal cells that release hydrochloric acid. Histamine binds to H2 receptors on these cells, turning the acid pump on. Ranitidine competes with histamine for those receptors, keeping the pump off and lowering acid levels. Less acid means fewer symptoms of gastroesophageal reflux disease (GERD) and faster healing of peptic ulcers.
Typical Uses and Dosage Forms
Before the 2020 recall, ranitidine was sold in several strengths and formats:
- Tablets - 75mg, 150mg, and 300mg
- Oral solution - 150mg/5ml
- Injectable - 50mg/ml for hospital use
Common dosing guidelines (for adults) were:
- Heartburn relief: 150mg twice daily or 300mg once daily after meals.
- GERD maintenance: 150mg twice daily, taken 30minutes before meals.
- Peptic ulcer treatment: 150mg twice daily for 4‑8weeks.
Pediatric dosing was weight‑based, usually 1‑2mg/kg per dose, not exceeding adult limits.
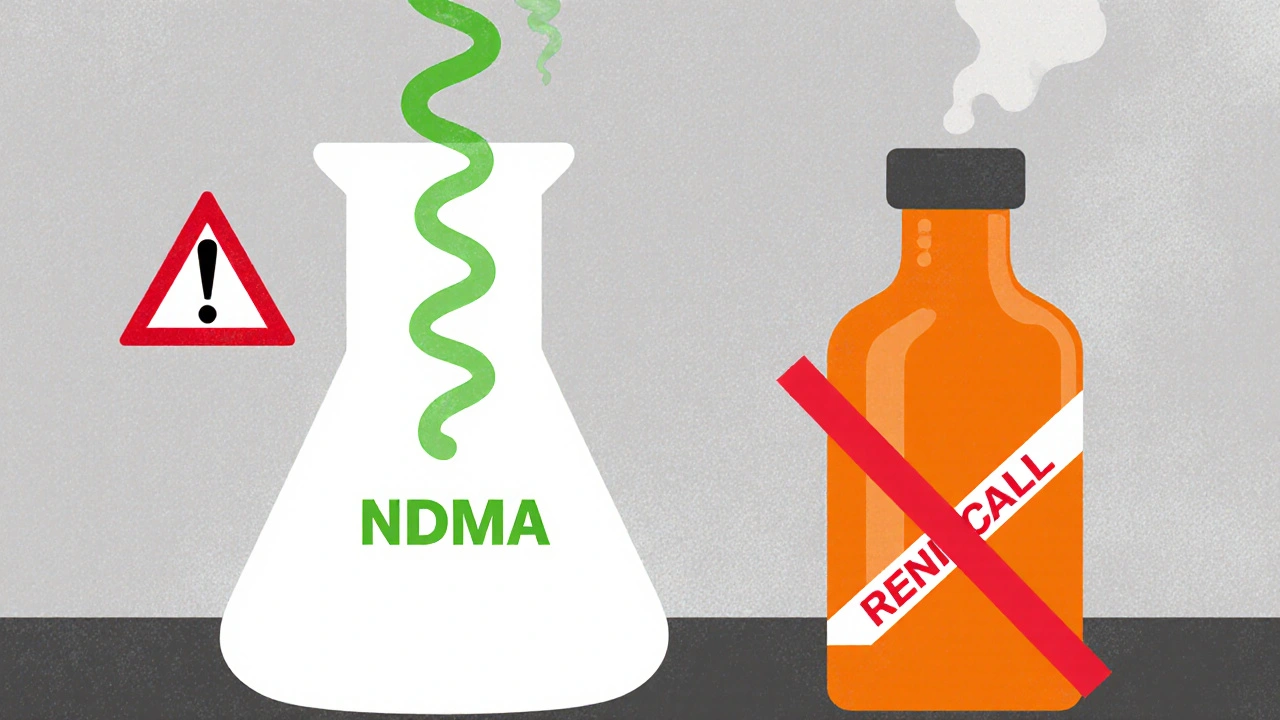
The NDMA Controversy and Global Recalls
In 2019, scientists discovered that ranitidine can break down into N‑nitrosodimethylamine (NDMA) under certain conditions-heat, humidity, and even normal storage. NDMA is a probable human carcinogen, meaning it could increase cancer risk if consumed over long periods.
Regulatory agencies responded:
- U.S. Food and Drug Administration (FDA) issued a voluntary recall in October2020 and later a final market removal.
- European Medicines Agency (EMA) followed with a similar withdrawal in November2020.
- Health Canada and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) also suspended ranitidine sales.
The key takeaway? Even if you have leftover tablets, the safest move is to stop using them and talk to a pharmacist or doctor.
Alternatives to Ranitidine - What’s Available Now?
If you need an acid‑reducing medication, you have two main families to choose from:
- Other H2 blockers: famotidine (Pepcid), cimetidine (Tagamet). They work the same way but have different safety profiles.
- Proton pump inhibitors (PPIs): omeprazole (Prilosec), esomeprazole (Nexium), pantoprazole (Protonix). PPIs block the final step of acid production, providing stronger, longer‑lasting suppression.
Here’s a quick side‑by‑side look:
| Attribute | Ranitidine | Famotidine | Cimetidine |
|---|---|---|---|
| Typical dose (adult) | 150mg BID | 20mg BID | 300mg BID |
| Onset of action | 30‑60min | 30‑60min | 45‑90min |
| Duration | 8‑12h | 10‑12h | 6‑8h |
| Key side effects | Headache, nausea | Diarrhea, constipation | Gynecomastia, drug interactions |
| NDMA risk | Yes (withdrawn) | No | No |
| Availability (2025) | Withdrawn worldwide | OTC & prescription | Prescription only |
Famotidine is the most popular replacement because it’s widely available over the counter, has a low interaction potential, and carries no NDMA concerns.
How to Switch Safely
If you were on ranitidine and need a new regimen, follow these steps:
- Consult your prescriber or pharmacist. Explain the recall and any lingering symptoms.
- Choose an alternative based on your condition:
- For occasional heartburn, try famotidine 20mg once daily after meals.
- For chronic GERD, famotidine 20mg BID or a low‑dose PPI (e.g., omeprazole 20mg daily).
- Give the new drug at least one week before judging effectiveness. Acid‑related symptoms can take time to settle.
- Monitor for side effects. If you notice new headaches, abdominal pain, or unusual fatigue, contact a healthcare professional.
Most people transition without trouble, but a short adjustment period is normal.

Common Questions About Ranitidine (and What to Do Now)
Below are the top concerns people still raise, even after the recall.
- Do I need to throw away my old ranitidine? Yes. Keep the medicine in a sealed bag and discard it according to local pharmacy guidelines.
- Can a short course of ranitidine be safe? Short‑term use still carries NDMA risk, and regulations advise complete avoidance.
- Is famotidine as effective for ulcers? Clinical studies show famotidine heals duodenal ulcers at similar rates to ranitidine when given in standard doses.
- Are PPIs better than H2 blockers? PPIs provide stronger acid suppression, which is helpful for severe GERD or erosive esophagitis. However, long‑term PPI use has its own concerns (e.g., magnesium deficiency, bone health). Choose based on symptom severity and doctor advice.
- What about NDMA in other drugs? So far, only ranitidine tablets and liquid have shown measurable NDMA formation. Ongoing monitoring continues for other medicines.
Key Takeaways
- Ranitidine was an H2 blocker that reduced stomach acid by blocking H2 receptors.
- NDMA contamination led to a global recall in 2020; the drug is no longer marketed.
- Safe alternatives include famotidine, cimetidine, and various proton pump inhibitors.
- Switching is simple: consult a pharmacist, pick a suitable alternative, and monitor your symptoms.
Frequently Asked Questions
Is it safe to keep ranitidine tablets at home?
No. Even unused tablets may contain NDMA, so the safest option is to discard them according to local pharmacy disposal rules.
How quickly does famotidine start working?
Famotidine typically begins to relieve heartburn within 30‑60 minutes, similar to ranitidine.
Can I use a PPI if I only have occasional heartburn?
For occasional symptoms, an H2 blocker like famotidine is usually enough. PPIs are reserved for frequent or severe reflux.
What should I do if I experience new stomach pain after stopping ranitidine?
Contact your healthcare provider. It could be a rebound increase in acid, which may be managed with a short taper or a different medication.
Are there any long‑term risks associated with famotidine?
Famotidine is generally well‑tolerated. Rarely, it can cause headaches, dizziness, or mild liver enzyme changes, but serious long‑term risks are uncommon.


9 Comments
Honestly, the ranitidine recall feels like a media circus that turned a tiny glitch into a full‑blown apocalypse, complete with doom‑laden headlines and panic‑selling. The molecule itself is fine; it’s the hype that’s truly toxic.
Got your point, and while the scare was big, the safety steps helped many avoid potential issues. It’s good we have alternatives now.
When we contemplate the ethical dimensions of pharmaceutical vigilance, we must acknowledge that the collective conscience of society demands unwavering transparency. 📜 The NDMA incident, while scientifically substantiated, became a crucible for moral reckoning. 🧭 It forced regulators to confront the latent perils lurking within seemingly benign tablets, exposing a chink in the armor of complacency. 🌐 Moreover, the public’s alarm was not merely a reactionary tremor but a profound yearning for accountability. 🍂 The discourse around ranitidine, therefore, transcends biochemistry; it delves into the philosophy of trust between healer and patient. 🏛️ If we surrender to fear without scrutiny, we betray the very principle of reasoned medicine. 🤔 Conversely, dismissing legitimate concerns as mere hysteria undermines the sanctity of informed consent. ✨ The balance lies in measured vigilance, where data guides policy, not sensationalism. 📊 As we pivot to alternatives like famotidine or PPIs, we must ensure that the lessons learned are etched into the regulatory fabric. 🛡️ Let us honor the victims of oversight by fortifying the safeguards for future generations. 🌱 In the grand tapestry of health, each thread-be it a molecule or a statute-must be woven with care and conscience. 🔗 Thus, the saga of ranitidine is a testament to the perpetual dance between innovation and responsibility. 🌙
hey folks i think its cool how everyone shares tips i just wanted to add that many people find famotidine works just fine for occasional heartburn its also pretty easy to get over the counter and i guess that makes the transition less stressful for most users
Honestly who cares about the tiny details.
In many cultures the act of swallowing a medicine is wrapped in ritual and trust, so the ranitidine debacle felt like a breach of that sacred pact. It reminds us that health decisions are not just clinical but also narrative, shaped by stories passed down through families. Even though the chemistry is universal, the way we react to recalls differs from one community to another, often colored by local folklore about poisons and cures.
Don't be fooled by the official narrative; the NDMA scare was a staged distraction to keep us dependent on foreign pharma giants. The real toxin is the silent agenda pushing us toward the next generation of chemically engineered drugs that will enslave our bodies. Wake up and see the pattern – every "safety" recall follows the same script, designed to usher in a new wave of control. Our ancestors trusted the earth, not synthetic lab concoctions, and that's the wisdom we need to reclaim.
While the article provides a comprehensive overview, it omits a critical appraisal of the pharmacoeconomic impact of transitioning patients from ranitidine to newer agents. A rigorous cost‑benefit analysis should be incorporated, as well as a discussion of potential drug‑drug interactions associated with long‑term PPI use. Moreover, the safety profile of famotidine warrants a more nuanced examination, given emerging data on rare hepatic effects.
Wow, another fancy report, because we totally needed more paperwork.